Introduction: Despite excellent outcomes for young patients with cHL, older patients (pts) age≥ 60 years have less favorable outcomes with a 5-year PFS of about 60% (Cheng, et al. Blood Advances 2022). This disparity is attributed to disease biology, comorbidities, treatment toxicity, dose reductions, and interruptions. Brentuximab vedotin (BV) is increasingly being used in the front-line setting for older pts. The aim of this study was to assess real-word outcomes with BV-based regimens in newly diagnosed older pts with cHL.
Methods: We retrospectively reviewed older pts age ≥60 consecutively diagnosed with cHL between 03/2018-01/2023. Data on demographics, medical comorbidities, AEs, PFS and OS were extracted from electronic medical records and were evaluated. For a historical cohort, we reviewed older pts consecutively diagnosed with cHL between 01/2014-03/2018, representing the pre-brentuximab era. Matching 1:1 (the BV cohort to the historical cohort) by age, sex and ECOG was performed.
Results: Forty-two patients were treated with BV-based regimens. Patient characteristics included median age of 72 years (range 60-88.5), 71% male, a median Cumulative Illness Rating Scale-Geriatric comorbidity score of 4 (range 0-10), 71% stage IV disease, 61% B symptoms, 49% EBV positive and had 54% had a non-nodular sclerosis histology, table. Twenty-seven (64%) patients received sequential BV-AVD; the rest either received co-current BV-AVD (9,21%) or other BV-palliative combinations (6,15%).
The most common adverse events were peripheral neuropathy (35%), infections (16%), and deconditioning (12%), leading to a 33% reduction in treatment, primarily brentuximab vedotin. Twelve patients (28%) did not complete planned treatment, five of them due to AEs, four due to disease progression and three due to other co-morbidities.
At median follow up of 2.4 years (95%CI 2.2-2.5), 2-year PFS and OS were 74% and 81%, respectively. After excluding patients treated with palliative regiments (n=36), 2-year PFS and OS were 76% and 93%, respectively. Seven patients died: four from other comorbidities, two from disease progression and one from AEs.
Numerical difference in 2-year PFS favoring sequential vs. concurrent BV-AVD was observed, not significant possibly due to low number of pts receiving BV-AVD.
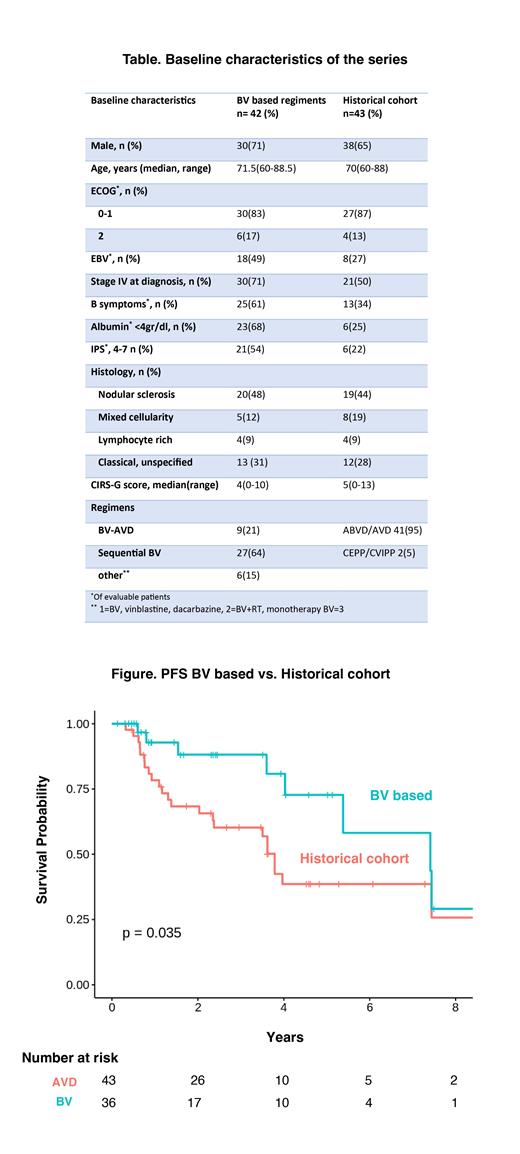
When compared to the historical cohort of 43 older patients treated with AVD-based regimens, pts treated with non-palliative BV-based regimens had significantly improved PFS (2-year PFS 65% vs 80%, p=0.035) ,figure. However, there was no statistical significance difference in OS (2-year OS 93% vs 94%, p=0.46). In the BV-based group, all relapsed pts were treated with anti-PD-1-based therapies. In contrast, among the AVD-based group, salvage treatment involved BV-based therapy for 74% of patients, PD-1 therapy for 39%, and chemotherapy with subsequent ASCT for 13%.
Conclusions: In this real-world series, sequential BV-AVD regimen was well-tolerated and safe with outcomes that are favorable and comparable to results from the phase II study by Evens et al (JCO 2018). Pts treated with BV-based regimens had significantly better PFS compared to the historical cohort, supporting the use of BV combinations for this elderly population. The lack of statistical significance in OS may be related to death from other comorbidities, limited sample size and the nature of a retrospective data. Additionally, the number of lymphoma-related deaths was low indicating that a significant number of patients can be successfully salvaged, thus also impacting OS observation. Ongoing multicenter data collection efforts are in progress to address the impact of novel agents on treatment of older adults with HL.
Disclosures
Falchi:Genmab: Consultancy, Research Funding; Seagen: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; AstraZeneca: Consultancy; Genentech: Consultancy, Other: Advisory Board, Research Funding; Abbvie: Consultancy, Other: Advisory Board, Research Funding; Roche: Consultancy, Research Funding. Ghione:Kite, A Gilead Company: Research Funding; Kyowa Hakko Kirin: Consultancy; AstraZeneca Pharmaceuticals: Consultancy; Secura Bio: Consultancy. Hamlin:ADC Therapeutics: Consultancy. Horwitz:Seattle Genetics: Research Funding; Celgene: Research Funding; ADC Therapeutics: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; ONO Pharmaceuticals: Consultancy; Yingli Pharma Limited: Consultancy; Shoreline Biosciences, Inc.: Consultancy; SecuraBio: Consultancy; Cimieo Therapeutics: Consultancy; Auxilius Pharma: Consultancy; Abcuro Inc.: Consultancy; Crispr Therapeutics: Research Funding; Affimed: Research Funding; Takeda: Consultancy, Research Funding; Trillium Therapeutics: Consultancy, Research Funding; Millenium: Research Funding; Verastem/SecuraBio: Research Funding; Tubulis: Consultancy. Johnson:Myeloid Therapeutics: Consultancy. Kumar:Janssen: Consultancy; Adaptive Biotechnologies: Research Funding; Seattle Genetics: Research Funding; Loxo/Lily Oncology: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Research Funding; Kite Pharma: Consultancy; Celgene: Research Funding; BridgeBio: Current equity holder in publicly-traded company; Astra Zeneca: Consultancy, Research Funding; Abbvie Pharmaceuticals: Research Funding; Beigene: Research Funding. Lue:OncLive: Consultancy; Merck: Consultancy. Palomba:Smart Immune: Honoraria; Juno: Honoraria, Patents & Royalties; Cellectar: Honoraria; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; MustangBio: Honoraria; Novartis: Honoraria; GarudaTherapeutics: Honoraria; Kite: Honoraria; Ceramedix: Honoraria; BMS: Honoraria; Thymofox: Honoraria; Synthekine: Honoraria. Torka:ADC Therapeutics: Consultancy; Lilly USA: Consultancy; Seagen: Consultancy; Genmab: Consultancy; Genentech: Consultancy; TG Therapeutics: Consultancy. Vardhana:Koch Disruptive Technologies: Consultancy; Immunai: Consultancy. Zelenetz:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; None other than mutual funds (401K): Current equity holder in publicly-traded company; BMS: Consultancy, Honoraria; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria; Abbvie: Research Funding; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; SAB: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding. Salles:Kite/Gilead: Consultancy; BeiGene: Consultancy; ATB Therapeutics: Consultancy; Merck: Consultancy, Honoraria; EPIZYME: Consultancy; Loxo/Lilly: Consultancy; Debiopharm: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Genmab: Consultancy; BMS/Celgene: Consultancy; Molecular Partners: Consultancy; Owkin: Current holder of stock options in a privately-held company; Novartis: Consultancy; Nurix: Consultancy; Orna: Consultancy; Nordic Nanovector: Consultancy; Ipsen: Consultancy, Research Funding; Incyte: Consultancy; Janssen: Consultancy, Research Funding. Moskowitz:Bristol-Myers Squibb: Research Funding; Beigene: Research Funding; Merck: Honoraria, Research Funding; Seattle Genetics: Honoraria, Research Funding; Incyte: Research Funding; ADC Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal